Orserdu (elacestrant) is an oral medication approved by the U.S. Food and Drug Administration (FDA) for the treatment of a specific type of advanced breast cancer. It is designed for postmenopausal women and adult men with estrogen receptor (ER)-positive, HER2-negative, ESR1-mutated advanced or metastatic breast cancer that has progressed after at least one line of endocrine therapy. In the EMERALD trial, patients with the ESR1 mutation who took Orserdu had a median progression-free survival of 3.8 months, compared to 1.9 months for those on standard-of-care endocrine therapy. This means Orserdu reduced the risk of disease progression or death by 45% in this group
What is the cost of Orserdu and is financial assistance available?
Orserdu is a high-cost specialty drug. Its list price is approximately $21,000 to $24,000 for a one-month supply.
However, the actual cost to a patient can vary significantly based on several factors:
- Insurance Coverage: The amount paid out-of-pocket depends heavily on an individual’s prescription drug plan. Insurance companies may require prior authorization before covering the medication, a process where the insurer verifies the treatment is medically necessary.
- Patient Assistance Programs: To mitigate the high cost, the manufacturer, Stemline Therapeutics (a subsidiary of the Menarini Group), offers a patient support program called Stemline ARC.
- Co-Pay Savings: For eligible, commercially insured patients, a co-pay savings card is available, which can reduce the monthly cost to as low as $0, with an annual savings cap of $25,000.
- Distribution: Orserdu is a limited distribution drug, meaning it is only available through select specialty pharmacies.

How has Orserdu performed in the market since its launch?
Since its FDA approval in January 2023, Orserdu has demonstrated a strong market launch and significant uptake.
- Strong Sales Growth: The drug has performed better than initial expectations. U.S. sales data from 2023 shows a rapid increase, starting from approximately $0.9 million in February and rising to nearly $40 million by July of the same year.
- Projected Revenue: Sales for 2023 were expected to reach at least $175 million.
- Addressing an Unmet Need: Orserdu is the first and only therapy specifically approved to treat patients with ER+, HER2- breast cancer that harbors an ESR1 mutation. These mutations are a known driver of resistance to standard endocrine therapies and are found in up to 40% of patients with this type of metastatic breast cancer. This clear, targeted indication has fueled its adoption.
What are the main alternatives or competitors to Orserdu?
Orserdu has carved out a specific niche, but it faces competition from both existing treatments and other drugs in development.
- Primary Competitor (Injectable SERD): The main established competitor is fulvestrant (Faslodex), which was the only other approved Selective Estrogen Receptor Degrader (SERD) for many years. However, there are key differences:
- Administration: Orserdu is an oral, once-daily pill, offering greater convenience than fulvestrant, which is administered as an intramuscular injection.
- Generics: Faslodex now faces competition from generics, which has eroded its market share.
- Efficacy in ESR1: Orserdu’s approval is specifically for the ESR1-mutated population, where it showed a significant improvement in progression-free survival compared to standard-of-care treatments, including fulvestrant.
- Pipeline Competition (Oral SERDs): Several large pharmaceutical companies are developing their own oral SERDs, which could become direct competitors. These include:
- AstraZeneca: Camizestrant
- Roche: Giredestrant
- Eli Lilly: Imlunestrant
While some other candidates, like Sanofi’s amcenestrant, have been discontinued after trial failures, the oral SERD space remains competitive.
- Other Standard-of-Care Therapies: In the broader second- and third-line treatment setting, other options include aromatase inhibitors (letrozole, anastrozole, exemestane) or combination therapies.
How is Orserdu being promoted to doctors and patients?
The marketing strategy for Orserdu is highly focused and built around its unique position as a targeted therapy.
- Focus on the ESR1 Mutation: The core of the strategy is to establish a “test-and-treat” paradigm. The FDA’s concurrent approval of the Guardant360 CDx assay as a companion diagnostic is crucial. Marketing efforts encourage oncologists to test for ESR1 mutations in patients whose disease is progressing, thereby identifying the precise patient population for Orserdu.
- “First-and-Only” Messaging: Stemline and Menarini are marketing Orserdu as the “first and only” oral SERD and the first therapy to specifically address ESR1 mutations, highlighting its innovation after nearly two decades without new endocrine therapies for this patient group.
- Targeting Healthcare Professionals: Marketing is aimed at oncologists and breast cancer specialists through medical conferences, publications, and engagement with key opinion leaders.
- Patient Access and Support: The Stemline ARC program is a key marketing component. By helping patients navigate insurance and providing financial assistance, the company removes barriers to access, which is critical for a high-priced drug.
- Global Expansion: Following its approval in the U.S. and Europe, the Menarini Group is sub-licensing the drug for development and commercialization in other markets, such as China, to expand its global reach.
Who is eligible for treatment with Orserdu (elacestrant)?
Orserdu isn’t for every breast cancer patient; it’s a highly targeted therapy designed for a very specific group. Here’s a simple breakdown of the ideal candidate for this treatment:
- Specific Cancer Type: The patient must have ER-positive (ER+) and HER2-negative (HER2-) breast cancer. This is the most common subtype, where cancer growth is fueled by estrogen.
- Advanced Stage: This treatment is for cancer that is advanced or metastatic, meaning it has spread beyond the breast to other parts of the body.
- The Key Genetic Marker: The patient’s tumor must have an ESR1 mutation. This is a specific genetic change that helps the cancer resist other treatments.
- Not a First-Step Treatment: Orserdu is for patients whose cancer has progressed or stopped responding after at least one prior line of endocrine therapy (like an aromatase inhibitor).
- Identified Through Testing: The ESR1 mutation must be confirmed through a specific test, usually a simple blood test (liquid biopsy) or a tissue biopsy.
- Approved for Specific Demographics: It is indicated for postmenopausal women and adult men.
- An Oral Therapy Option: It is for patients who can take a daily oral medication.
How many patients could potentially benefit from Orserdu?
To understand the impact of a targeted therapy like Orserdu, it’s helpful to look at the numbers. While exact figures can fluctuate, we can estimate the size of the patient population in the U.S. and around the world who could be candidates for this treatment.
The Target Population in the United States
Here’s a step-by-step look at the numbers in the U.S.:
- ER+, HER2- Breast Cancer Cases: This is the most common subtype of breast cancer. About 70% of all breast cancers fall into this category.
- Patients with Metastatic Disease: It is estimated that approximately 6% to 10% of new breast cancer cases are metastatic from the start. Additionally, a significant number of patients with early-stage breast cancer will eventually develop metastatic disease. There are nearly 170,000 people in the U.S. currently living with metastatic breast cancer.
- The ESR1 Mutation Factor: Up to 40% of patients with metastatic ER+, HER2- breast cancer that has progressed on prior endocrine therapy will have an ESR1 mutation.
In which countries is the drug currently available?
Based on these figures, it is estimated that approximately 11,000 patients in the U.S. are diagnosed each year with tumors harboring ESR1 mutations after progressing on first-line treatments, making them potential candidates for Orserdu.
The Target Population at a Global Level
The numbers expand significantly when we look at the worldwide picture:
- Global Breast Cancer Incidence: Breast cancer is the most commonly diagnosed cancer worldwide, with over 2.3 million new cases diagnosed each year.
- Metastatic Cases Worldwide: Applying the same percentages, a substantial number of these cases are ER+, HER2- and will eventually become metastatic.
Estimated Global Target Population:
Globally, it’s estimated that around 100,000 patients could be eligible for treatment with an oral SERD like Orserdu based on having ER+, HER2- metastatic breast cancer with an ESR1 mutation that has progressed after endocrine therapy.
This highlights the significant number of patients worldwide who face the challenge of treatment resistance and could benefit from this targeted therapeutic approach.
Global Access: Where is Orserdu Available?
Since its groundbreaking approval, Orserdu (elacestrant) has been steadily making its way through regulatory processes around the world. For patients and their families, knowing where the drug is approved and available is crucial.
Here are the key markets where Orserdu has been given the green light:
- United States: The U.S. was the first to approve Orserdu. The Food and Drug Administration (FDA) gave its approval in January 2023, and the drug is fully available for prescribed patients who meet the specific criteria.
- European Union (EU): Orserdu received approval from the European Commission in September 2023. This centralized approval makes it available for marketing in all 27 EU member states. Key countries where it has since launched include:
- Germany
- The Netherlands
- Austria
- United Kingdom: Following its departure from the EU, the UK has its own regulatory body. The Medicines and Healthcare products Regulatory Agency (MHRA) granted Orserdu marketing authorization in Great Britain in November 2023. It has been available to eligible National Health Service (NHS) patients in England since December 2023.
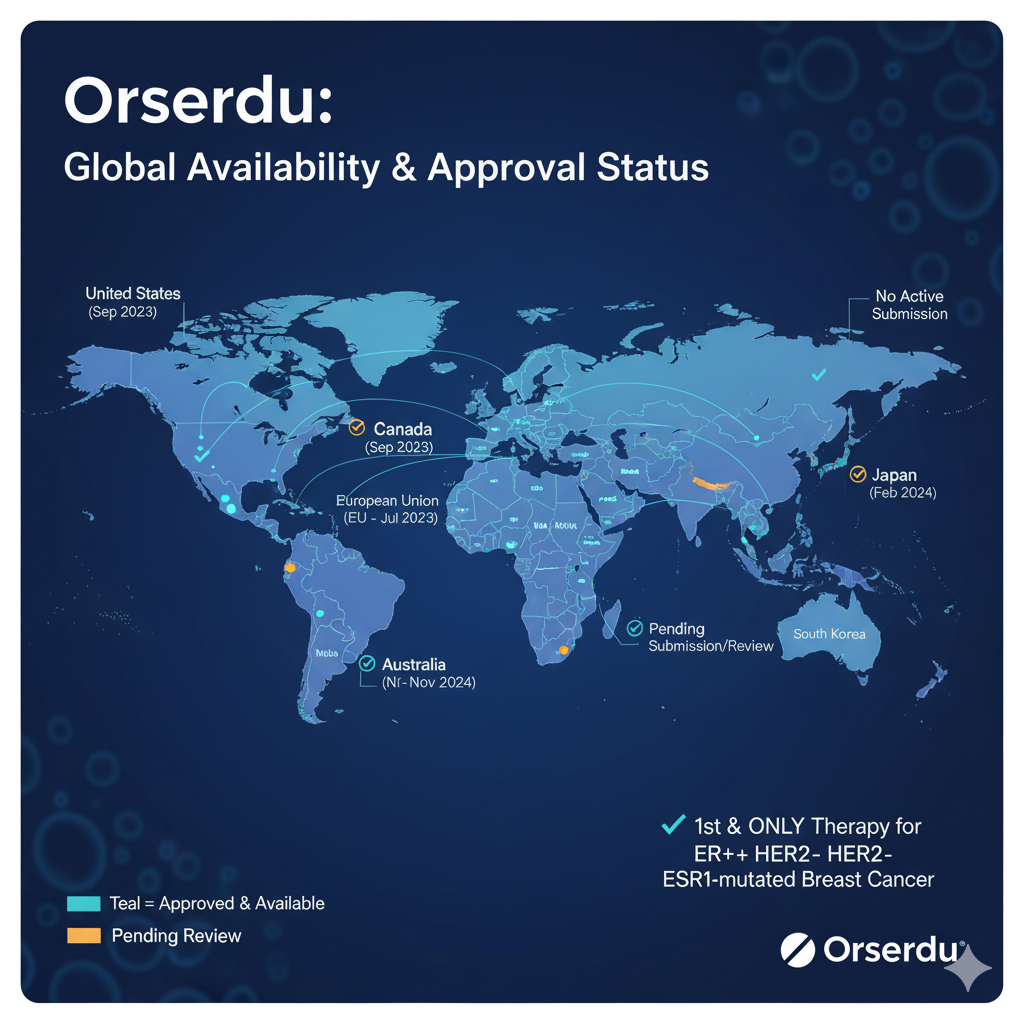
Expanding Availability:
The Menarini Group, which commercializes Orserdu, is actively working to expand its availability. While the initial launches have focused on major markets in North America and Europe, efforts are underway to secure approvals and reimbursement in other regions to make the therapy accessible to more eligible patients worldwide.
What are the future projections for 2025 and beyond?
Looking at 2025, the outlook for Orserdu remains positive as it continues to penetrate markets in the U.S. and expand globally.
- Sustained Growth Expected: Market analysts and the company itself anticipate continued sales growth in 2025. This is driven by increased physician awareness, broader insurance coverage, and its expansion into European and other international markets.
- A Key Player in a Growing Market: The overall market for this class of drugs (called SERDs) is expected to grow significantly. Market analysis suggests that Orserdu, along with other similar drugs in development, could collectively generate an estimated $3 billion in revenue by 2034 in the U.S. alone for metastatic breast cancer.
- Foundation for Future Therapies: Menarini Group is heavily invested in Orserdu’s future. The company is presenting data in 2025 from the ELEVATE study, which explores using Orserdu in combination with other targeted cancer drugs. Success in these trials could vastly expand its use and revenue potential in the years to come, positioning it as a foundational therapy in breast cancer treatment.


